
Quality Control Best Practices In The Pharmaceutical Distribution Chain
Quality Control in the pharmaceutical industry is essential to maintain the safety and efficacy of medicines, vaccines, and other pharmaceutical products. It demands strict adherence to quality assurance best practices during the manufacturing and distribution of pharmaceuticals.
Substandard medicines pose various health threats and can also tarnish the reputation of the healthcare provider. Therefore, healthcare entities must enforce stringent quality checks and exclusively partner with suppliers implementing the same to ensure better treatment outcomes and instil trust among the patient community.
Why Is Quality Control Essential In The Pharmaceutical Industry?
According to the WHO, 1 in 10 medical products in low and middle-income countries is substandard or falsified. Not only do these substandard pharmaceutical products pose a risk to patient health, but they also make the general population lose trust in medicines and healthcare systems. Substandard or counterfeit medicines may also pose threats such as antimicrobial resistance and can lead to significant financial losses for healthcare organizations in the long run.
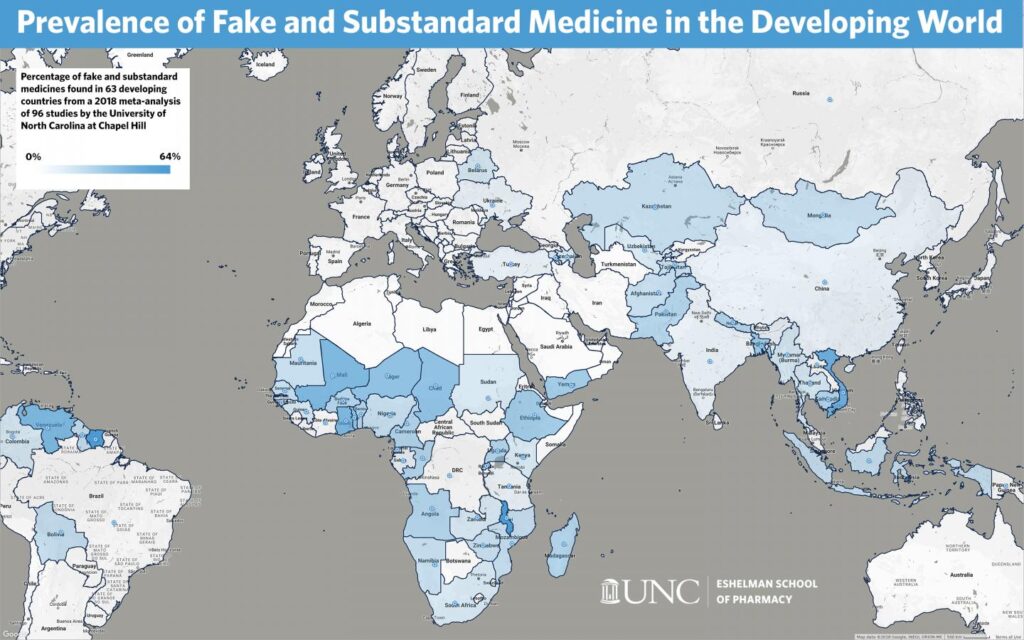
Image source – UNC Eshelman School of Pharmacy
The above figure represents the prevalence of substandard and fake medicines across developing countries, as analyzed by a study in 2018. It indicates that the problem of substandard medicines is the gravest in parts of Africa, South America, and the Middle East. These low and middle-income countries struggle the most with substandard pharmaceuticals because of complex supply chains, limited access to affordable medicines, and poor Quality Management Systems.
Therefore, healthcare entities working in these affected geographies must ensure that they partner with reputed pharmaceutical suppliers and distributors that strictly follow the recommended quality assurance best practices for the pharmaceutical distribution chain.
5 Quality Control Best Practices For Pharmaceutical Distribution
1. Accreditation of Suppliers
When selecting pharmaceutical suppliers, healthcare providers must ensure that they are compliant with the industry standards for the quality control of pharmaceutical products. Some parameters to assess include the physical condition of their warehouses and equipment, the qualifications of their staff, how up-to-date their tools and procedures are, and their SOPs to ensure quality control while handling pharmaceuticals.
By exclusively partnering with GDP-compliant distributors that source from facilities accredited by USFDA, EMEA, PIC/s and WHO, healthcare entities can provide high-quality medicines to their patients and eliminate the risk of counterfeit or substandard drugs.
2. Monitoring and Documentation of Product Quality
Healthcare organizations must ensure that the pharmaceutical supplier is running quality checks at each touchpoint of the distribution chain and documenting the same in a quality report. Furthermore, the supplier must source the drug from licensed GMP-compliant manufacturers and should have access to the quality documents maintained by the manufacturing facility. This could include reports of identity tests, assays, dissolution testing, impurity analysis, bioequivalence tests, stability testing and any other relevant test reports maintained by the manufacturer.
Apart from the above, healthcare entities may conduct some routine quality checks on samples at their end, such as inspecting the physical integrity of the packaging and the product, dissolution tests, spectrometric analysis, and quantitative assays to measure the active ingredients (APIs) in the medicine. These tests on random samples can further ensure the quality and efficacy of the medicine at the point of care.
3. Adherence to Good Storage and Distribution Practices
Storage and distribution are crucial components of the pharmaceutical supply chain. These steps may involve handling, repackaging, and transport of pharmaceutical products, which can potentially impact the quality of the medicines. To prevent this, pharmaceutical distributors must adhere to the Good Distribution Practices (GDP) for pharmaceuticals recommended by the WHO.
The storage facilities for pharmaceutical products should be clean and sanitary and the temperature and humidity should be maintained within the recommended limits for the medical products. Moreover, only authorized personnel should have access to the storage units, and the products should be handled carefully to prevent contamination or damage.
Additionally, the loading and unloading of pharmaceutical shipments must be done by trained staff to prevent any damage to the product or the packaging. Robust outer packaging materials must be used for these shipments to maintain the quality and integrity of the pharmaceuticals. Furthermore, reliable means of transportation that are compliant with the guidelines for pharmaceutical distribution must be deployed. The pharmaceutical distributor must maintain an efficient supply chain with the help of quality management systems, risk management strategies, trained personnel, and good documentation practices.
4. Temperature-Controlled Logistics
Temperature-sensitive pharmaceuticals must be transported in a temperature-controlled environment throughout the supply chain to maintain their quality and efficacy. To achieve this, pharmaceutical distributors may use active or passive cooling solutions, such as refrigerated vehicles and containers or insulated packaging with PCM-based cooling. A reliable cooling technology must be selected for each leg of the supply chain based on the desired temperature range and the transport duration. Furthermore, live-tracking of temperature through sensors integrated into the vehicles or packaging can ensure that the temperature limits are not breached at any point.
Besides temperature-controlled vehicles and packaging, ensuring quick loading and unloading of shipments, minimizing logistical delays through efficient planning, maintaining adequate environmental conditions at loading/unloading/storage junctions, and deploying secondary cooling technologies and backup power sources for emergencies can ensure seamless temperature control throughout the pharmaceutical supply chain.
5. End-To-End Traceability
End-to-end traceability refers to best practices to monitor and record the quality of pharmaceutical products throughout the supply chain. It starts with product authentication and serialization at the source, followed by frequent quality checks and live monitoring of the shipment throughout transport, using technologies such as GPS trackers for location tracking, and temperature sensors for temperature monitoring. This ensures that healthcare entities can get live information about their pharmaceutical shipment throughout its journey.
Supply chains in developing countries are often complex, with multiple touchpoints in the distribution chain. At each touchpoint, the distribution team must inspect the shipment and document key quality parameters. Furthermore, only authorized and trained personnel should be allowed access to these touchpoints to ensure that the shipment is handled with care and that any tampering by miscreants is prevented.
By maintaining end-to-end traceability throughout the distribution chain and performing quality checks at all touchpoints, pharmaceutical suppliers can ensure that they are delivering high-quality products to healthcare providers. Furthermore, any quality issue can be flagged early, traced to its root cause, and resolved in future shipments, ensuring that healthcare entities receive only the best-quality medicines.
Bottom Line
Substandard medicines pose a threat to patients and healthcare systems, particularly in low- and mid-income countries with complex supply chains and limited access to affordable pharmaceuticals. Therefore, healthcare entities in these geographies must partner with reliable global pharmaceutical suppliers that strictly implement quality assurance best practices throughout the pharmaceutical distribution chain. This will allow healthcare providers to offer safe and efficacious treatments to their patients and build the trust of the local community in the healthcare system.
At Eudaico, we comply with the Good Distribution Practices recommended by the WHO and source pharmaceutical products exclusively from facilities accredited by USFDA, EMEA, PIC/s and WHO. We have robust and efficient quality management systems in place to supply high-quality pharmaceuticals to different geographies across the globe.
So, if you are a healthcare entity looking for a trusted partner for reliable pharmaceutical supplies, feel free to contact us.


